
Hard rocks, such as Granite are so hard that water cannot permeate through, so rainwater will simply run-off the surface and absorb fewer minerals along the way, known as surface water. Areas with hard rock formation such as Cornwall, Wales or Scotland will naturally have softer water.
Inversely, soft rocks, such as Limestone are highly permeable and the water passes through, known as ground water. As the water passes through it absorbs minerals, such as calcium and magnesium. The more contact with soft rock formations the harder the water becomes.

Water hardness is typically measured in parts per million (ppm) and is categorised according to this scale:

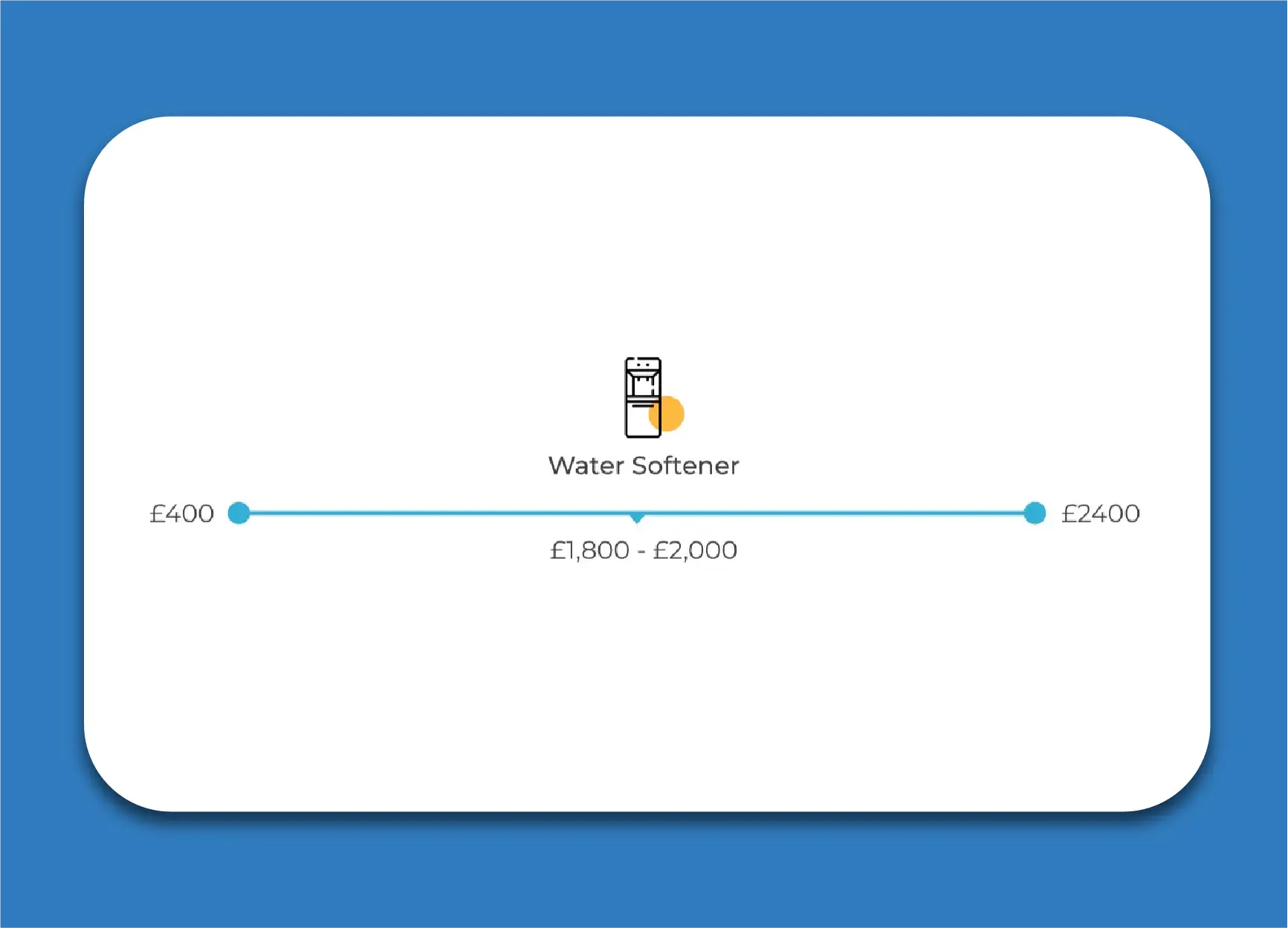
We are lucky to live in a beautiful area of the country, however due to the high levels of limestone and chalk, we have very hard water. In the area we cover we typically see water hardness ranging from between 280-320 PPM.



















By subscribing you agree to with our Privacy Policy and provide consent to receive updates from our company.